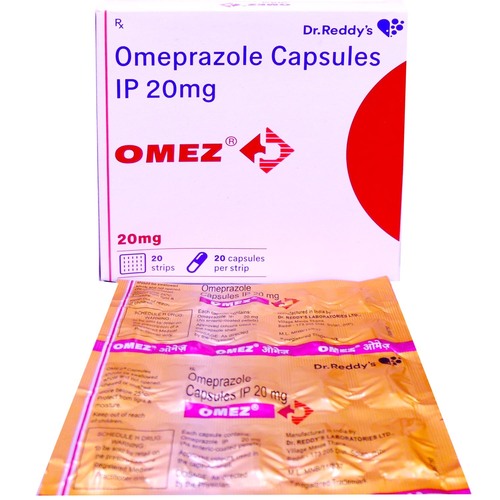
Dexlansoprazole Delayed Release Tablet
0.66 - 0.79 USD ($)/Unit
Product Details:
- Drug Type General Medicines
- Ingredients Dexlansoprazole Delayed Release 30mg, 60mg Tablet
- Physical Form Tablets
- Function Other
- Recommended For Antacid, Antiulcer
- Dosage AS DIRECTED BY PHYSICIAN
- Dosage Guidelines As Per Directed By Physician
- Click to View more
X
Dexlansoprazole Delayed Release Tablet Price And Quantity
- 0.66 - 0.79 USD ($)/Unit
- 500 Unit
Dexlansoprazole Delayed Release Tablet Product Specifications
- Suitable For All
- General Medicines
- Antacid, Antiulcer
- Tablets
- Other
- 1000 Unit
- Store at Dry and Cool Place.
- As Per Directed By Physician
- AS DIRECTED BY PHYSICIAN
- Dexlansoprazole Delayed Release 30mg, 60mg Tablet
Dexlansoprazole Delayed Release Tablet Trade Information
- Nhavasheva Port, Mundra Port, Hajira Port.
- Western Union Letter of Credit (L/C) Telegraphic Transfer (T/T) Cash in Advance (CID) Cash Advance (CA)
- 10000 Unit Per Day
- 5-7 Days
- Yes
- If order is confirmed we will reimburse the sample cost
- jar packing
- Australia Eastern Europe Central America Middle East South America Asia Western Europe North America Africa
- All India
- WHOGMP/GMP/WHOc-GMP FDA Approved. ISO certified EUGMP & PICs Approved
Product Description
Important Safety Information
- DEXILANT is contraindicated in patients with known hypersensitivity to any component of the formulation. Hypersensitivity reactions, including anaphylaxis, have been reported. Acute interstitial nephritis has been reported with other proton pump inhibitors (PPIs), including lansoprazole. Discontinue DEXILANT if acute interstitial nephritis develops.
- PPIs, including DEXILANT, are contraindicated with rilpivirine-containing products.
- In adults, symptomatic response with DEXILANT does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing.
- PPI therapy may be associated with increased risk of Clostridium difficile associated diarrhea.
- Long-term (1 year) and multiple daily dose PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the conditions being treated.
- New onset or worsening of cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE) have been reported in patients taking PPIs. The majority of PPI-induced lupus erythematosus cases were CLE. Avoid administration of PPIs for longer than medically indicated. Discontinue DEXILANT and refer the patient to an appropriate specialist for evaluation, if signs or symptoms of CLE or SLE occur. Most patients improve with discontinuation of the PPI alone in 4 to 12 weeks.
- Daily treatment with any acid-suppressing medications over a long period of time ( > 3 years) may lead to malabsorption or a deficiency of cyanocobalamin (Vitamin B-12).
- Hypomagnesemia has been reported rarely with prolonged treatment with PPIs.
- Serum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acidity which may cause false positive results in diagnostic investigations for neuroendocrine tumors. Temporarily stop DEXILANT treatment 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high.
- Concomitant use of PPIs with methotrexate may elevate and prolong serum concentrations of methotrexate and/or its metabolite, possibly leading to toxicity. With high dose methotrexate administration, consider a temporary withdrawal of DEXILANT.
- PPI use is associated with an increased risk of fundic gland polyps that increases with long-term use, especially > 1 year. Use the shortest duration of PPI therapy appropriate to the condition being treated.
- DEXILANT is not recommended in pediatric patients less than two years of age. Nonclinical studies in juvenile rats with lansoprazole have demonstrated an adverse effect of heart valve thickening. Dexlansoprazole is the R-enantiomer of lansoprazole.
- Most commonly reported adverse reactions in adults were diarrhea (4.8%), abdominal pain (4.0%), nausea (2.9%), upper respiratory tract infection (1.9%), vomiting (1.6%), and flatulence (1.6%).
- The adverse reaction profile in patients age 12 to 17 years was similar to adults. The most commonly reported adverse reactions in patients age 12 to 17 years 5% were headache, abdominal pain, diarrhea, nasopharyngitis, and oropharyngeal pain.
- Decreased exposure of some antiretroviral drugs (e.g., rilpivirine, atazanavir, and nelfinavir) when used concomitantly with DEXILANT may reduce antiviral effect. Avoid concomitant use of nelfinavir with DEXILANT. Increased exposure of other antiretroviral drugs (e.g., saquinavir) when used concomitantly with DEXILANT may increase toxicity of the antiretroviral drugs.
- Patients taking concomitant warfarin may require monitoring for increases in international normalized ratio (INR) and prothrombin time (PT). Increases in INR and PT may lead to abnormal bleeding and even death.
- DEXILANT may interfere with absorption of drugs for which gastric pH is important for bioavailability (e.g., digoxin, iron salts, erlotinib, dasatinib, nilotinib, mycophenolate mofetil [MMF], ketoconazole/itraconazole). Use DEXILANT with caution in transplant patients receiving MMF.
- Concomitant tacrolimus use may increase tacrolimus whole blood concentrations.
- A hyper-response in gastrin secretion in response to the secretin stimulation test may falsely suggest gastrinoma. Temporarily stop DEXILANT treatment 30 days before assessing to allow gastrin levels to return to baseline.
- Avoid concomitant use of DEXILANT with St. John's Wort or rifampin due to decreased exposure of DEXILANT.
- No studies have been conducted in patients with severe hepatic impairment (Child-Pugh Class C). The use of DEXILANT is not recommended for these patients.
Indications
DEXILANT (dexlansoprazole) 30 mg and 60 mg delayed-release capsules are indicated in patients age 12 years for:
- Healing all grades of erosive esophagitis (EE) for up to 8 weeks
- Maintaining healing of EE and relief of heartburn for up to 6 months in adults and up to 16 weeks in patients age 12 to 17 years
- Treating heartburn associated with symptomatic non-erosive gastroesophageal reflux disease (GERD) for 4 weeks
Use of DEXILANT in patients age 12 to 17 years is supported by evidence from adequate and well-controlled studies of DEXILANT capsules in adults with additional safety, efficacy and pharmacokinetic data in patients age 12 to 17 years.
The safety and effectiveness of DEXILANT have not been established in patients < 12 years of age.
Other Products in 'Anti Ulcer, GIT Agent & Antacid ' category
Get in touch with us